On 13-16 June 2022, the European Innovation Council (EIC) brought together 20 EIC-backed SMEs, start-ups and scale-ups with the objective to showcase impactful biotech technologies at BIO 2022 in San Diego, USA. Among the participants, Biohope stood out for its unique laboratory solutions that contribute to the treatment of conditions associated with chronic inflammation. In this interview, Isabel Portero, CEO and medical doctor, talks about her career path, the challenges that companies are facing today and her personal experience about how bold ideas can be turned into reality by the help of the EIC in order to improve the delivery of healthcare services.
Biohope investigates, develops and commercialises unique laboratory testing solutions to personalise medical treatment of chronic inflammatory conditions. What was your motivation behind the creation of the company and how difficult was the process of transforming your innovative idea into reality?
I am a medical doctor, so I have had the chance of experiencing the problem we solve personally. From decades we have used corticoids and immunosuppressant drugs to tamper the immune system to treat chronic inflammation, autoimmune diseases and prevent rejection in organ transplantation. These drugs are prescribed empirically (trial/error) and also following rigid protocols which end up with a “coffee for all”. The consequences imply a significant burden, in terms of overimmunosuppression (cause of severe infections and cancer) and underimmunosuppression (uncontrolled disease – inflammation or rejection). Physicians are currently blinded when prescribing these potent medications, and everybody is assuming the consequences. The clinical, societal, and economic problem associated is huge, as more than 150 million patients suffer from these conditions and a significant morbimortality is associated with over/under immunosuppresion.
There is a clear need for tools to personalise medical treatment in this area. Such a tool would revolutionize the management of autoimmune diseases and transplantation at the same level as Precision Oncology, nowadays. We need to move towards Precision Immunology.
Can you explain what your novel diagnostic tool, IMMUNOBIOGRAM, is about?
Immunobiogram is a cell technology functional assay, regulatory classified as In Vitro Diagnostic Test, which measures the response of patient circulating immune cells to a panel of commonly used immunosuppressant medication and corticoids.
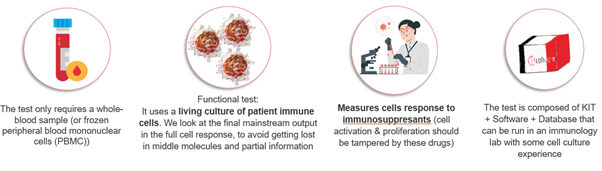
The response measured is activation/proliferation of cells, which is inhibited by immunosuppresant drugs. The more potent is the drug over patient cells, the more efficacy is expected when the drug is administered. The response can also be described as “sensitivity” to medication: when a patient shows more sensitivity to a drug, it means that the drug is more potent for that patient than other.
We have consistently demonstrated that sensitivity to drugs is linked to the relevant clinical outcomes, like rejection and inflammation.
The test is to be used by the physicians as an informative tool to better select the medication for each individual patient, choosing drugs in which sensitivity is higher to obtain the desired effect over the clinical condition, but adapting dosages in order to avoid undesired side effects associated to overimmunosuppression.
You are also a biotech R&D scientist, founder and CEO of Biohope with a multifaceted background. What inspired you to follow a career in the biotechnology sector?
As a medical doctor and R&D scientist, I wanted to help patients by developing novel useful tools for the clinical practice. Medicine and Biotechnology work together when Biotechnology is used as a tool to deliver innovation to healthcare.
Precision Medicine is one of the principal axis of modern Medicine and I wanted to follow that start in order to make a contribution to patients, doctors and society.
The empowerment of women entrepreneurs and innovators constitutes a top priority for Europe. Could you briefly share your views on the existing gender gap in science and what would you recommend as possible actions of overcoming it? How can the EIC contribute to this?
I am 52 years old and I have quite a number of stories of invisibility, put in the background, paternalism, complaisance etc.. in my professional career. I think that for young people things are now different, at least somewhat different that in the past. Fortunately, this has evolved positively.
Nevertheless, there is a non-written rule about entrepreneurship and taking risks in which women are systematically underscored. Maternity is one of the key factors and also, uncertainty associated with careers out of the comfort of big companies or public jobs.
Science is great, but you have to pay the bills and take care of your children. In a startup company, many things can occur, not all under your control. Many are exciting and good, others are bad, so there is a strong pressure in the teams to make projects survive and not being terminated at the middle of the game even when things evolve satisfactory. Healthcare biotech is a long run and time needs money. This is a reality for all biotech companies in Europe.
EIC instruments are very good tools to give oxygen to good projects during the “death valley” of 8-12 years between R&D is started and the company is self-sustainable. In America, this is covered by VCs, but in Europe the investment environment is very different and not so supportive. Both quantities and criteria are different. Because of this, EIC has been of great help to companies that without the European support would have never developed. Nevertheless, some adjustments maybe required, because some schemes, as blended funds etc., rely on fundraising from VCs, which is precisely the obstacle many start-up companies face in Europe.
As a female leader, what has been the most significant barrier in your professional life?
For a long time, only few people saw me as a leader. The majority had a vision of a good technical person without the personal characteristics needed to be a real leader.
I had to believe in myself as a starting point, with little help at the beginning.
Previously, you were selected to exhibit your innovation at BIO 2022 under EIC Overseas Trade Fairs Programme 2.0. How do you evaluate your participation and overall experience?
I found it very useful and interesting. US is a key market for all healthcare biotech companies and this has been a huge opportunity to know people and start alliances.
How should a biotech start-up approach corporations and institutions in order to forge business partnerships globally, especially in trade fairs such as BIO?
You need to work a lot in advance:
- Have a clear map of attendants. Classify and select your target companies and persons.
- Work for an interview during the fair, be clear about the objective of the meeting, what you offer and what you seek and always do a post-fair follow up.
- Make strong walking days at the Fair and introduce yourself to all you did not reach previously that you are interested in.
The pandemic has drastically changed the way startups do business. What do you think the impact of COVID-19 has had on the biotechnology industry?
The impact has been good, because now even my mother knows what a PCR is, that mRNA vaccines exist, and therefore, they have some flavor about what Biotechnology is about. This should imply a stronger support to Biotech companies because it has been clearly shown what we can deliver to the world. But not with the economic recession we are facing, you never know.
Finally, is there any insightful advice that you could offer to potential applicants?
It is worth attending the BIO International Convention. Attending under the European Commission umbrella, poses a significant opportunity to show your business.


